Mutographs
Discover how unusual patterns of mutation are induced by different cancer-causing events


Professor Sir Mike Stratton, Team Lead
Director, Wellcome Sanger Institute
Mutographs
INSTITUTIONS
5
LOCATIONS
UK, France, USA
FUNDED BY
Cancer Research UK - £20m
SPECIALISMS
Genetics, chemistry, bioinformatics, epidemiology
Detective work: searching for unknown causes of cancer
It’s a cliché, but prevention really is better than cure. By examining the damaging fingerprints left on our DNA by cancer-causing factors, the Mutographs team hopes to identify unknown causes of cancer to help prevent more people from developing the disease.
Combing for clues
Funded by:
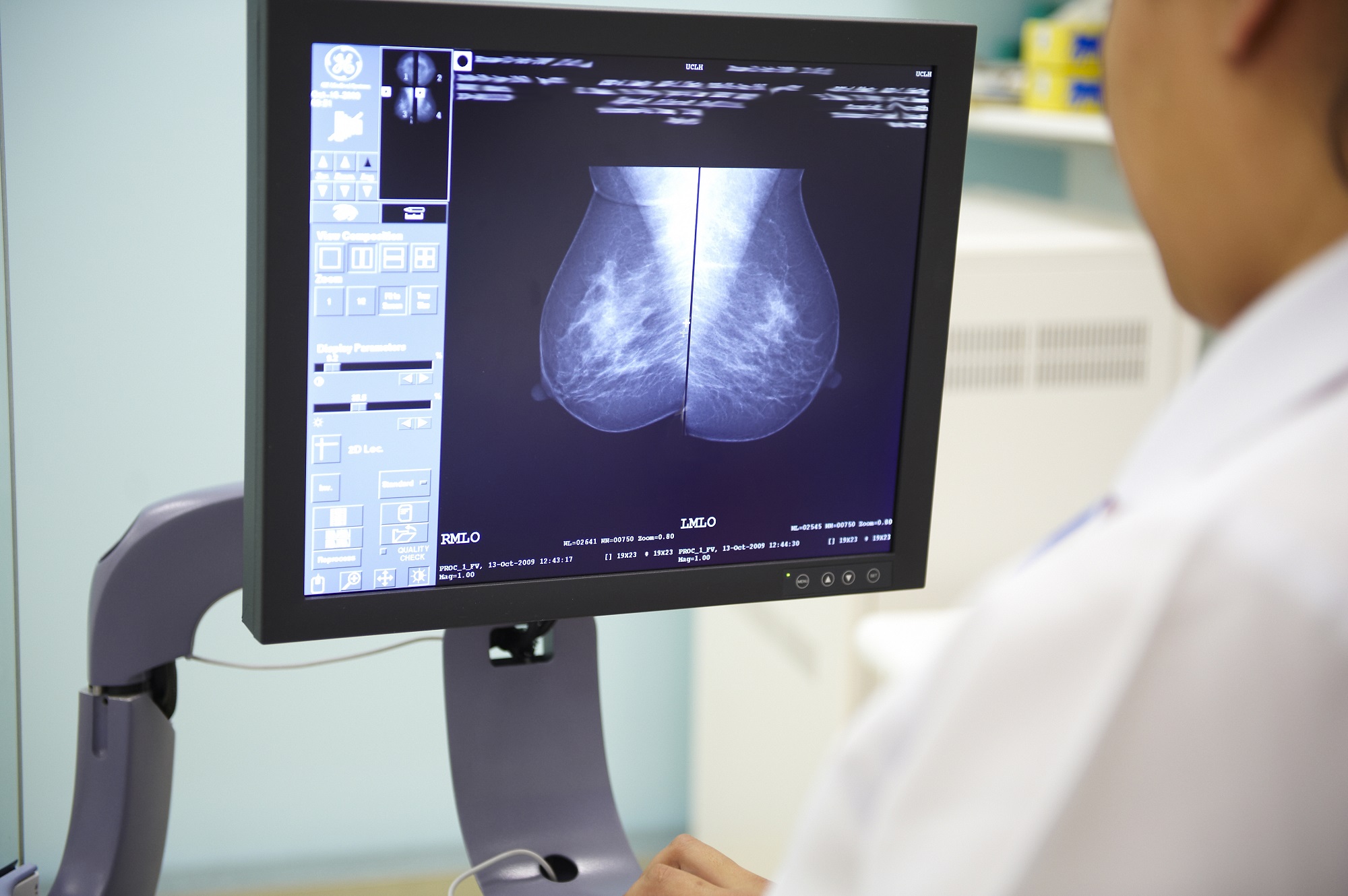
As a cell goes through life, its DNA picks up a unique pattern of damage. This can be caused by exposure to factors like UV light, from behaviours like smoking or drinking alcohol, or can happen randomly through natural cellular processes. Depending on the amount and type of damage, this can cause a cell to become cancerous.
Working backwards from this damage can provide clues as to the cause of cancer: each factor will leave a different, distinctive pattern, known as a ‘mutational fingerprint’. For example, cancers caused by UV radiation will have a different fingerprint to those linked to tobacco.
So far, scientists have uncovered 50 cancer-associated mutational fingerprints. But they can only trace around half of them back to a specific cause. Figuring out what causes the other 25 or so cancer-associated fingerprints could identify new, potentially preventable causes of cancer.
Taking on this enormous challenge is the Mutographs team, a diverse group of experts from the UK, US and France. Together, they hope to dramatically improve our understanding of what causes cancer and prevent more cases in the future, by helping people reduce their risk of the disease.
Collecting the evidence
Mutographs’ ambition is of epic scale, with samples collected across 5 continents, from 5,000 people with either pancreatic, kidney, oesophageal or bowel cancer. Importantly, these people are from countries with either high or low levels of these cancers. For example, one type of oesophageal cancer is 10–20 times more common in Iran and East Africa than in other countries.
But what drives this massive difference? To answer, the team will identify the mutational fingerprint of each sample, before cross-referencing between each cancer type and against countries with high or low levels of the disease.
The team hopes to determine which fingerprints are more common – or potentially only present – in patients’ samples in countries with high levels of specific cancers, and which are less common – or even absent – in countries with low levels. From here, they’ll study the habits, lifestyles and environments of patients who’ve generously donated their samples, to determine what caused these fingerprints and if these factors could be avoided in future.

Professor Sir Mike Stratton, Team Lead
Director, Wellcome Sanger Institute
Our aim is to help prevent more cancers and reduce the global burden of this disease.
Filling in the gaps
By harnessing the power of discovery, Mutographs could dramatically improve our understanding of what causes cancer. If successful, it could lead to better information for people looking to reduce their own risk of cancer and help inform government policies, making our ability to prevent certain cancers much more effective.
Detective skills
As well as working backwards to identify unknown cancer causes, another important aim of Mutographs’ work is to determine whether suspected cancer-causing factors are responsible for specific fingerprints, using animal models and organoids (mini lab-grown organs) made from human cancer cells. The team is also studying people thought to be at risk of cancer to better understand cancer development.
Mike Stratton trained in medicine specialising in histopathology. His primary research interests have been in the genetics and genomics of cancer. He mapped and identified the breast cancer susceptibility gene BRCA2, discovered BRAF mutations in malignant melanoma and described the mutational signatures of DNA damage and repair processes in cancer genomes.
Organisation
Wellcome Sanger Institute

Allan Balmain was born in Wick, Scotland, studied Organic Chemistry at the University of Glasgow, and carried out post-doctoral work France and Germany. He subsequently worked in the University of Glasgow and Cancer Research UK Scotland Institute (formerly the Beatson Institute), before moving to the United States in 1997. Balmain established a molecular link between chemical carcinogen exposure and initiation of tumour development in 1983, and showed that the types of mutations found in oncogenes depend on the causative chemical agent. A major focus has been the links between genetic and environmental factors and how they interact to cause cancer susceptibility.
Organisation
University of California, San Francisco
Discipline
Cancer Genetics

Ludmil Alexandrov is an Assistant Professor at University of California San Diego, where his research focuses on understanding mutational processes in human cancer and human ageing through the use of mutational signatures.
Ludmil was born in Sofia, Bulgaria, studied computer science at Neumont University, and completed his graduate work with Prof Sir Mike Stratton at the University of Cambridge and the Wellcome Trust Sanger Institute. During his PhD, Ludmil developed the first comprehensive map of the mutational signatures in human cancer. He also held a distinguished Oppenheimer Fellowship at Los Alamos National Laboratory.
Ludmil’s role in the Grand Challenge will be the development of novel computational approaches for understanding and explaining cancer risk through mutational signatures.
Organisation
University of California San Diego
Discipline
Computational Biology

Paul Brennan runs the Genetics Section at the International Agency for Research on Cancer (IARC) in Lyon. The primary aim of the Genetics Section is to use genetics and genomics techniques to (i) understand the causes of cancer, and (ii) identify ways to detect and treat cancers at earlier stages.
In the Mutograph Grand Challenge he will coordinate the large international effort of recruiting approximately 5000 cancers across 5 continents for 5 different cancers types.
Organisation
International Agency for Research on Cancer
Discipline
Epidemiology

Dr Peter Campbell is Head of Cancer Genetics and Genomics at the Wellcome Trust Sanger Institute, having started a Wellcome Trust Senior Clinical Fellowship in 2010. He completed specialist training in Haematology in New Zealand and Australia in 2002. Following this, he completed a PhD at the University of Cambridge in the molecular pathogenesis of myeloproliferative disorders. Since 2007, Dr Campbell has been employed at the Cancer Genome Project, Wellcome Trust Sanger Institute.
His major interest is cancer genomics, and in particular genome-wide analyses of somatic mutations in tumours. The four major areas of interest have been: the discovery of new cancer genes; the identification of somatic mutation processes operative in tumours; the characterisation of patterns of cancer evolution and the translation of these fundamental insights about cancer biology into better management of patients.
Organisation
Wellcome Sanger Institute
Discipline
Cancer Genetics

David H. Phillips is Professor of Environmental Carcinogenesis at King’s College London, where he leads a research team investigating the properties and mechanism of action of agents known or suspected to cause cancer.
His role in the Grand Challenge will be to expose human tissue organoids in culture to different agents in order to generate mutational signatures, which will then be compared with the signatures in human tumours and carcinogen-induced animal tumours, shedding new light on the aetiology of human cancer.
Organisation
King's College London
Discipline
Environmental Carcinogenesis

Mimi McCord founded the charity now known as Heartburn Cancer UK following the death of her husband in 2002. He died aged 47, nine weeks after being diagnosed with oesophageal adenocarcinoma. Mimi is now a lay member of NICE and BSG guideline groups focusing on Barrett’s oesophagus and OAC, to improve diagnosis, treatment and management. Additionally she sits on an expert panel advising the DH on a public oesophago-gastric cancer awareness campaign.
Mimi works determinedly to raise awareness of the link, and risk factors, between heartburn, Barrett’s and OAC within the public domain and medical profession.

Maggie Blanks was a carer for her husband Alan when he was diagnosed with pancreatic cancer at the age of 55 in 2002. After his death in 2003, and having discovered how little research was then being done into pancreatic cancer, she set up the charity Pancreatic Cancer Research Fund (PCRF) to raise new money for research. Since its start, PCRF has funded £7m of research and promoted the need for more research by the major funders.

%20(3).png)
.jpg)